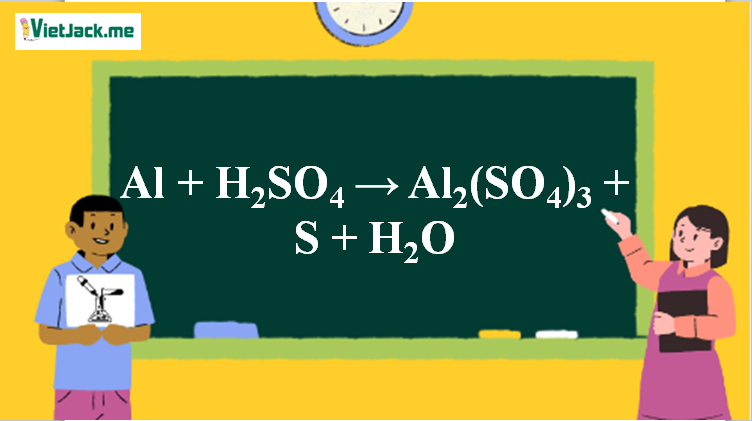
High Quality Chemical Formula Al2 So4 3.18H2O Aluminum Sulfate of Aluminum - China Aluminium Sulfate, Aluminium Sulfate 17% Min | Made-in-China.com
ALUMINIUM SULPHATE practical iron free Extra Pure | Lab chemical distributors, Lab chemicals exporter, Laboratory chemical suppliers, Lab chemical supplier, Laboratory Chemicals, Laboratory chemicals manufacturer, Lab chemical manufacturer, Alpha ...
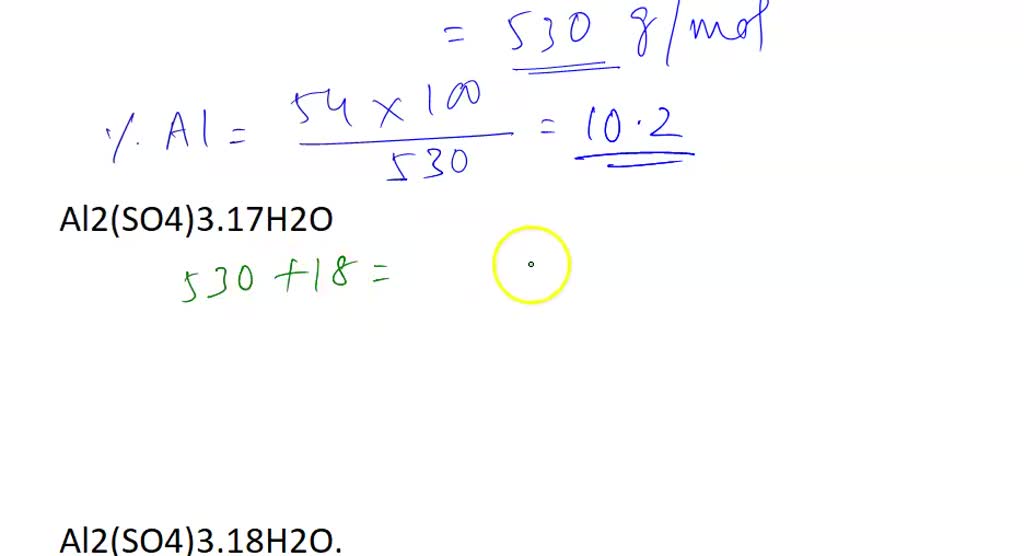
SOLVED: Aluminum sulfate occurs as a hydrate, commonly with 16-18 moles of H2O. Calculate the molar masses and Al% for each Al2(SO4)3*16H2O, Al2(SO4)3*17H2O, and Al2(SO4)3*18H2O.

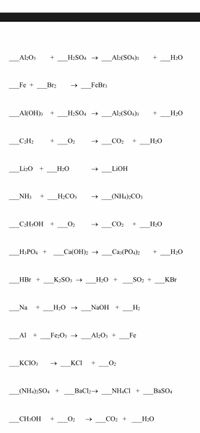
Consider the reaction of Al2O3 withH2SO4 to form Al2(SO4)3 and H2O. If 3.84 g AL2O3 is reacted with excess - brainly.com

SRL Aluminium Sulphate Octadecahydrate extrapure, 98%, 500Gm, CAS NO 7784-31-8, Molecular Formula : Al2(SO4)3.18H2O, Storage : Room Temperature Shelf Life : 60 Months for laboratory and industrial use : Amazon.in: Industrial & Scientific

Jual Aluminium (III) Sulfat, 18-hidrat | Al2(SO4)3.18H2O 500 gram - Kota Bandung - Rofa Laboratorium Centre | Tokopedia

Which of the following is not isomorphous with true alum and is called pseudo alum? (A) FeSO4.Al2(SO4)3.24H20 (B) K2SO4.Al2(SO4)3.24H2O (C) K2SO4.Cr2(SO4)3.24H20 (D) (NH4)2SO4.Fe2(SO4)3-24H20
:no_upscale()/3000.jpg)
19516.3000 - Aluminium sulfate solution, 40 % Al2(SO4)3 * 18 H2O/l, technical grade, 1 L | Analytics-Shop



![Aluminium Sulfate Octahydrate [Al2(SO4)3.8H2O] Molecular Weight Calculation - Laboratory Notes Aluminium Sulfate Octahydrate [Al2(SO4)3.8H2O] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/aluminium-sulfate-octahydrate-molecular-weight-calculation-300x178.jpg)