
Balance the following chemical equation H2O2+O3⇒H2O+O2 Indicating the changes in oxidation numbers of oxygen, the equivalent weight of H2O2 this reaction.

SOLVED: Equations: MnO4 + H2O2 + H2O -> MnO2 + O2 + H2O 2 MnO4 + 6 H2O2 + 5 H2O -> 2 MnO2 + 5 O2 + 8 H2O MnO4 + 8 H+ + Se -> MnO2 + 4 H2O O2 + 3 O2 + 2 e Mn7+ + 2 e -> Mn2+ Mn7+ + Se -> Mn2+

2H202 alkaline medium *2H20 + 02 the proposed mechanism is as given below : (1) H2O2 +1 → H2O+IO (slow) (2) H202 + 10 + H20+1+02 (fast) (i) Write rate law the
I) H2O2 + O3 → H2O + 2O2 (II) H2O2 + Ag2O → 2Ag + H2O + O2 Role of hydrogen peroxide in the - Sarthaks eConnect | Largest Online Education Community

Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect

Kinetic studies on the reaction of 2 with H2O2 in buffered MeCN/H2O... | Download Scientific Diagram
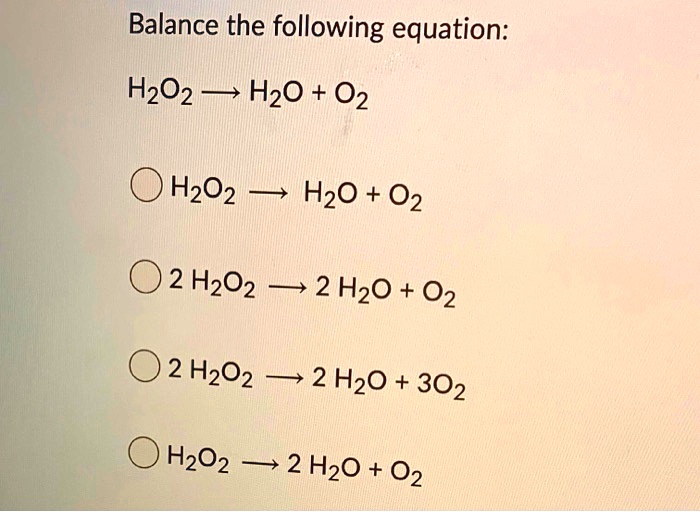
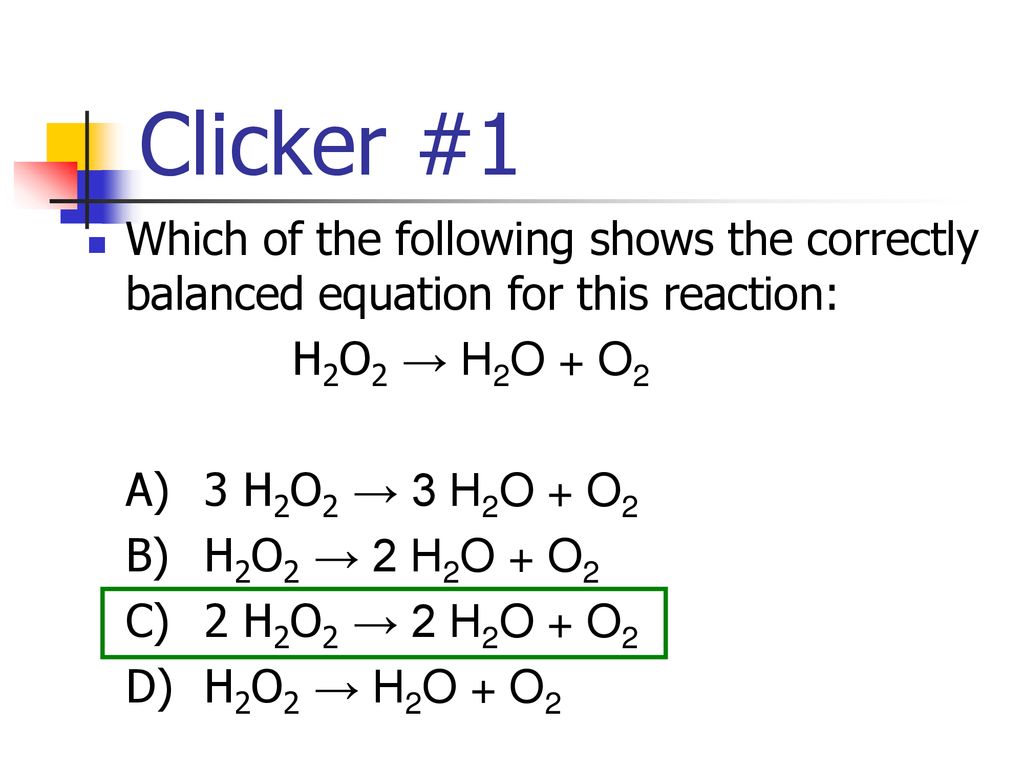
SOLVED: Balance the following equation: H2O2 H2O + O2 H2O2 H2O + O2 2 H2O2 2 H2O + O2 2 H2O2 2 H2O + 3 O2 H2O2 2 H2O + O2

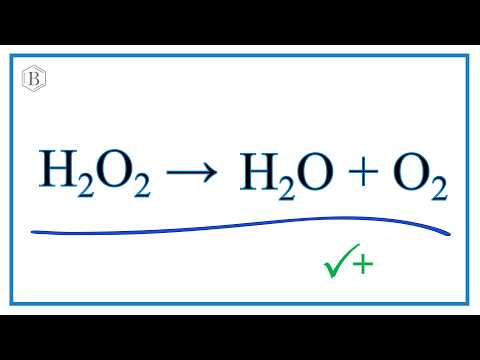
Comment équilibrer : H2O2 → O2 + H2O (peroxyde d'hydrogène, dioxygène, eau) | Physique-Chimie - YouTube

H2O2 is decomposed to H2O and O2 in following sequence of reaction. 1)H2O2(aq)+ I (aq) + H2O(0) + OI (aq) 2)H2O2(aq) +Ol'(aq) → H2O0) + O2(g) a) Write the chemical equation overall
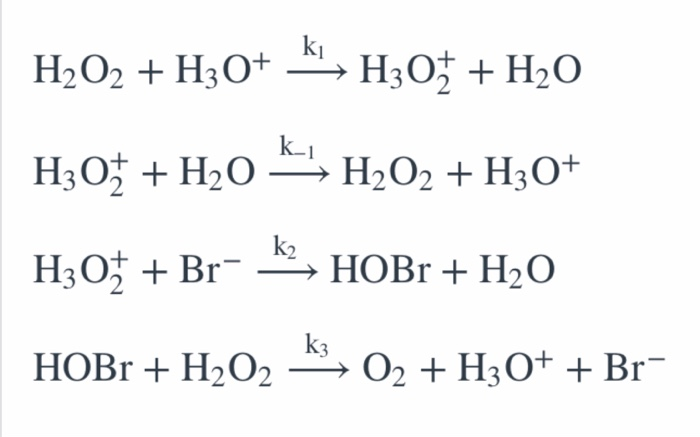





![SOLVED] I H2O2+O3→H2O+2O2II H2O2+Ag2O→2Ag+H2O+O2Role of hydroge - Self Study 365 SOLVED] I H2O2+O3→H2O+2O2II H2O2+Ag2O→2Ag+H2O+O2Role of hydroge - Self Study 365](https://static.tllms.com/ckeditor_assets/pictures/29060/content_1.jpg)






