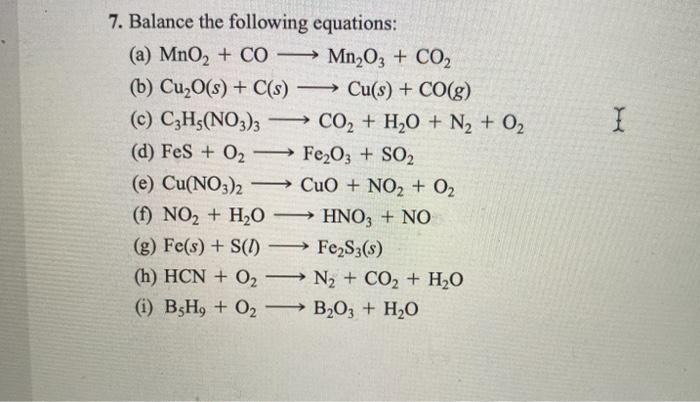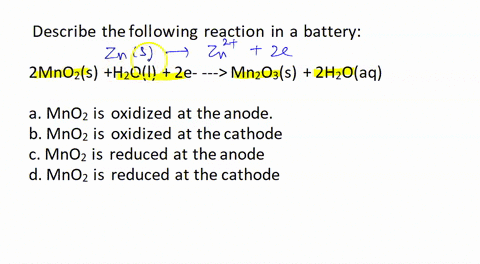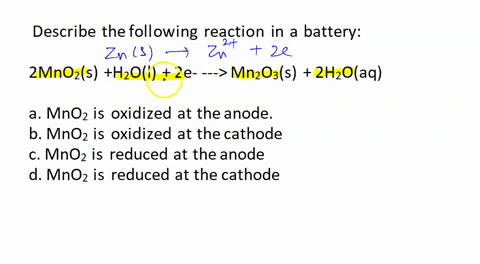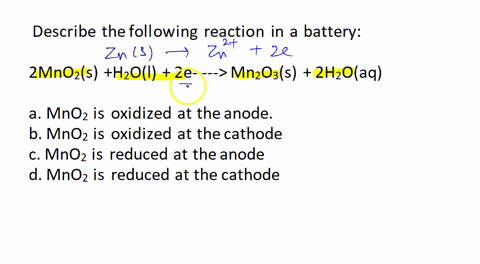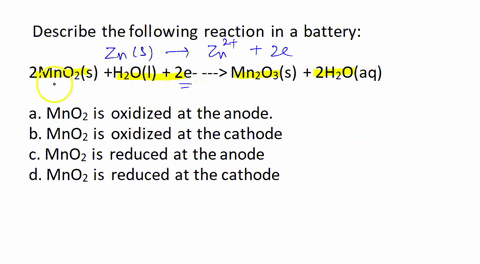
SOLVED: Describe the following reaction in a battery: 2 MnO2(s) + H2O(l) + 2e- —> Mn2O3(s) + 2 H2O(aq) a. MnO2 is oxidized at the anode. b. MnO2 is reduced at the
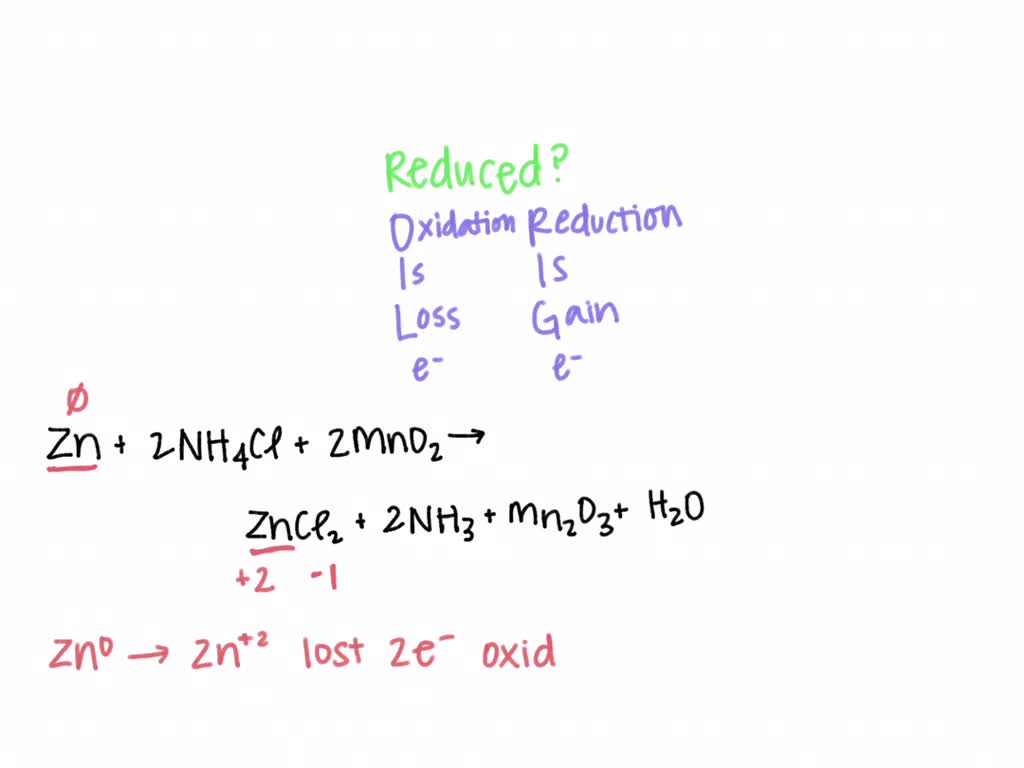
SOLVED: In an acid dry cell battery for which the reaction is Zn + 2 NH4Cl + 2 MnO2 ? ZnCl2 + 2 NH3 + Mn2O3 + H2O, what atom is reduced?
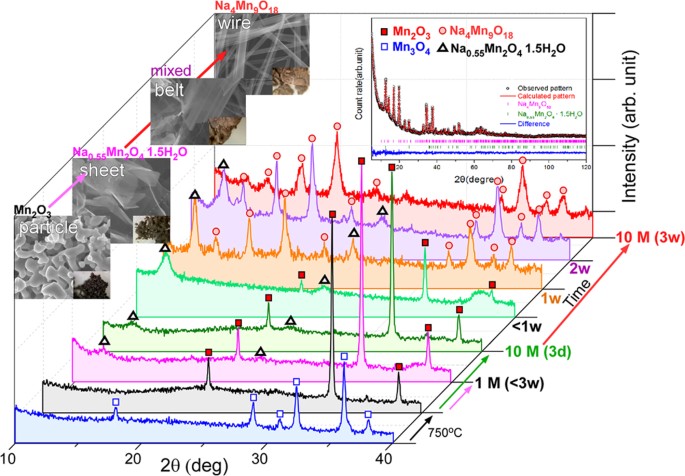
Understanding hydrothermal transformation from Mn2O3 particles to Na0.55Mn2O4·1.5H2O nanosheets, nanobelts and single crystalline ultra-long Na4Mn9O18 nanowires | Scientific Reports
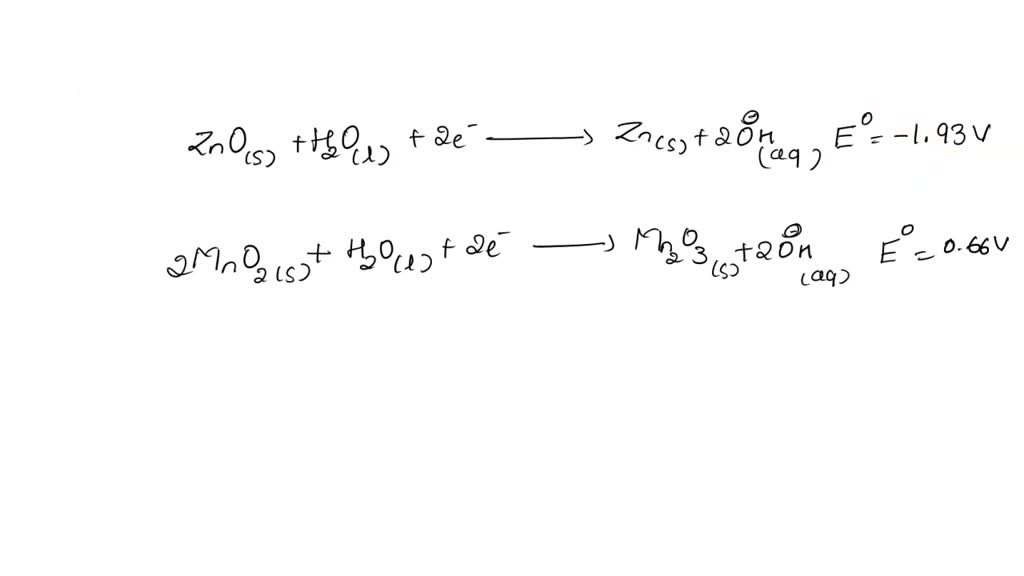
SOLVED: The standard reduction potentials of the half-reactions in single-use alkaline batteries are: ZnO(s) + H2O(l) + 2e- –> Zn(s) + 2OH-(aq) Eo = -1.93V 2MnO2(s) + H2O(l) + 2e- –> Mn2O3(s) +
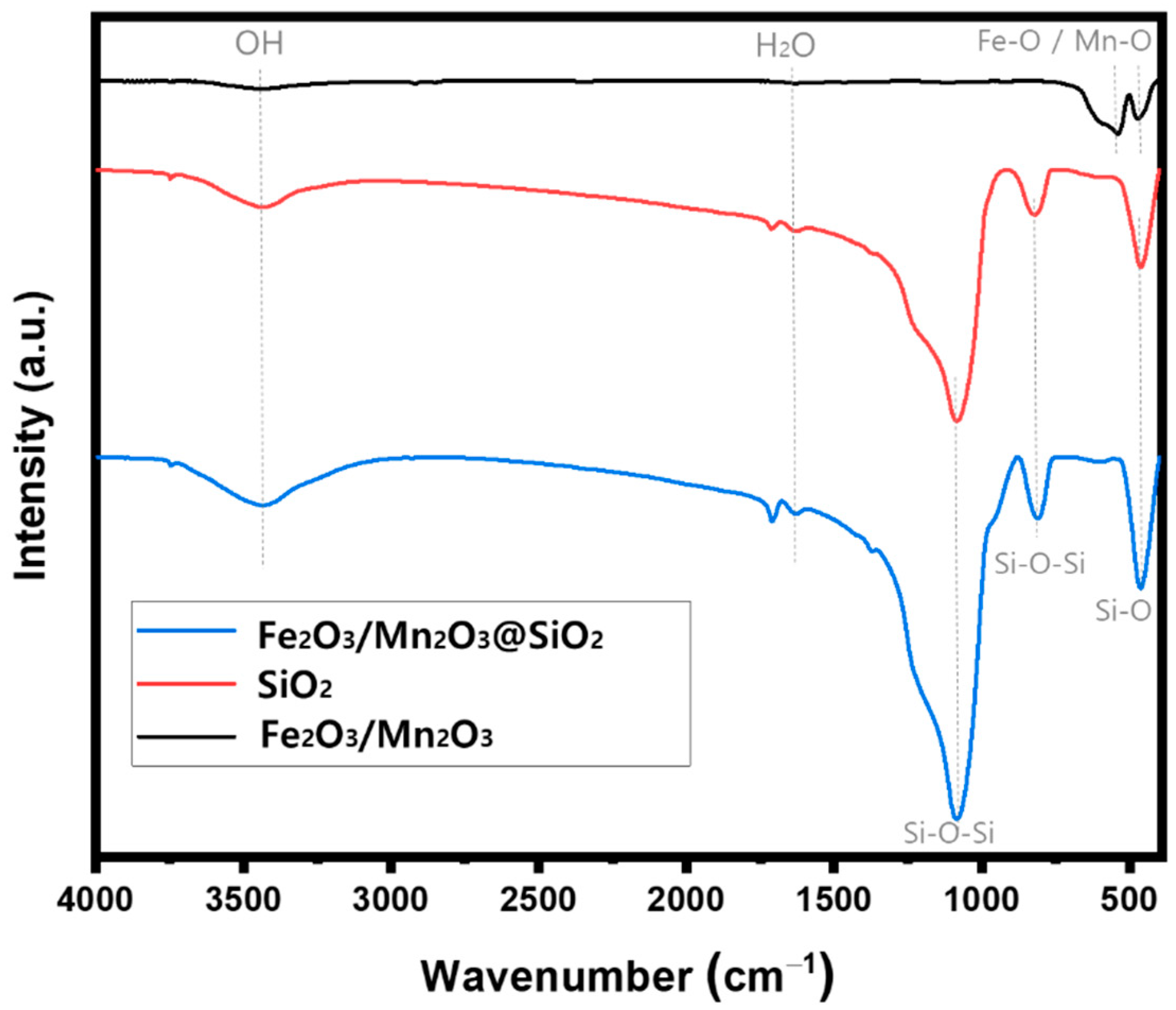
Catalysts | Free Full-Text | Synthesis of Fe2O3/Mn2O3 Nanocomposites and Impregnated Porous Silicates for Dye Removal: Insights into Treatment Mechanisms

Understanding hydrothermal transformation from Mn2O3 particles to Na0.55Mn2O4·1.5H2O nanosheets, nanobelts and single crystalline ultra-long Na4Mn9O18 nanowires | Scientific Reports

The Most Active Oxidase‐Mimicking Mn2O3 Nanozyme for Biosensor Signal Generation - Chen - 2021 - Chemistry – A European Journal - Wiley Online Library
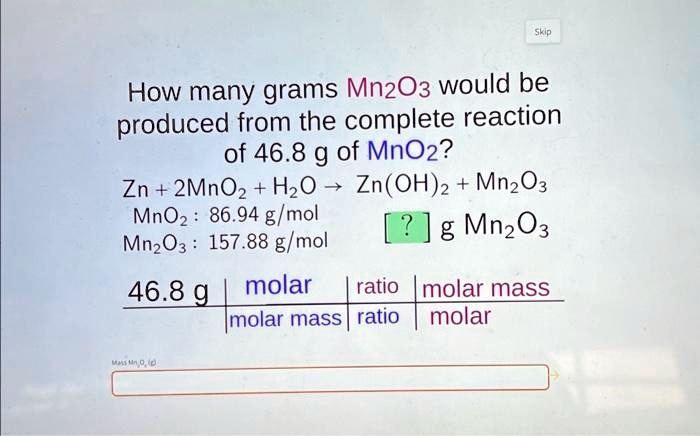
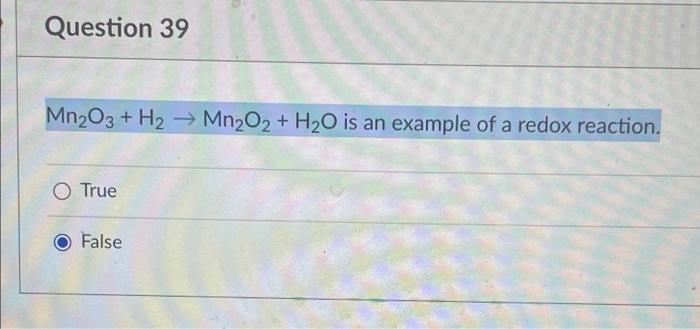
SOLVED: How many grams of Mn2O3 would be produced from the complete reaction of 46.8 g of MnO2? Zn + 2MnO2 + H2O -> Zn(OH)2 + Mn2O3 MnO2: 86.94 g/mol Mn2O3: 157.88

![Solved [3] Manganese dry cell Cathode 2MnO2 + 2H+ + 2e → | Chegg.com Solved [3] Manganese dry cell Cathode 2MnO2 + 2H+ + 2e → | Chegg.com](https://media.cheggcdn.com/study/b83/b839ebec-1828-4793-8e66-88ddd87ee165/image)


![How many grams Mn2O3 would be produced from the complete reaction of 46.8 g of M || 102 [algebra] How many grams Mn2O3 would be produced from the complete reaction of 46.8 g of M || 102 [algebra]](https://p16-ehi-va.gauthmath.com/tos-maliva-i-ejcjvp0zxf-us/33c8cdf452324a458870f75e4227d3cb~tplv-ejcjvp0zxf-webp.webp)